Couple participates in COVID-19 clinical trials
Story by Wendi Hawthorne (Kunkel) '13

Class of 2013
It is hard to put into words how frightening it is when the very thing you have avoided for the better half of a year finds its way into your home.
When I think back to my days as an SFA student reporter for The Pine Log and my short stint as a small-town newspaper reporter, I never imagined the role I would play in writing one of the more important stories of my career. Just seven months after landing my dream job in public affairs covering infectious diseases and public health at The University of Texas Health Science Center at Houston, a global pandemic emerged, and I became the source for a patient perspective from the front lines of this public health emergency.
From the onset of the pandemic, I was in daily communication with researchers and physicians about the latest findings on COVID-19, trying to distribute critical health information to the public in my role as a media relations specialist. So, when my husband, Brandon, wasn't feeling well one October afternoon after learning a few of his colleagues had tested positive for COVID-19, it was time to put that knowledge into action for both of us.
Brandon quickly scheduled an appointment for a rapid test. When he called an hour later to tell me he had tested positive for COVID-19, my heart sank. It is hard to put into words how frightening it is when the very thing you have avoided for the better half of a year finds its way into your home.
The next day I was still feeling normal — no fever or body aches, and my sense of smell and taste were both still intact. I made an appointment to be tested that afternoon so I could know my status for peace of mind. I tested negative, so we continued to quarantine apart in our home.
The month prior to Brandon's illness, I began working with a research team at McGovern Medical School at UTHealth to help educate the public about two clinical trials testing the effectiveness of monoclonal antibodies. It dawned on me — Brandon and I were both qualified candidates for these studies. Brandon fit the requirements for ACTIV-2, a study on multiple treatments, including a monoclonal (laboratory-made) antibody, at preventing mild COVID-19 from advancing to severe illness in the outpatient setting.
While I was not positive for COVID-19, I met the criteria for the REGN-COV2 trial, studying if a combination antibody treatment can prevent COVID-19 illness in individuals who live with someone with the virus. When we dialed the research hotline and I told the research coordinator on the other end of the phone I was actually reaching out to make an appointment to assess us for these two studies, I had to laugh. "The irony of this is not lost on me," I said.
My first introduction to working in health care public relations was during my days at SFA. As an undergraduate student studying journalism, I had the opportunity to intern in the community relations department at Nacogdoches Memorial Health. It was only meant to be a summer internship before my senior year, but it turned into a yearlong role — one I am so grateful for.
Because of that experience, I knew when I entered the workforce that I wanted to work for an organization whose mission is helping others. I desired a career that would allow me to use my love of writing to inform others and to engage with the community where I lived and worked.
Nearly eight years later, I know my education at SFA prepared me well to chase that dream. Guided by professors who taught me to ask the important questions, find the story and communicate it in a way that is meaningful to the public, I gained confidence that has certainly placed me on the path I am following today.
Rooted in that same passion to make a difference for the community, Brandon and I decided we would both enroll in the respective clinical trials.
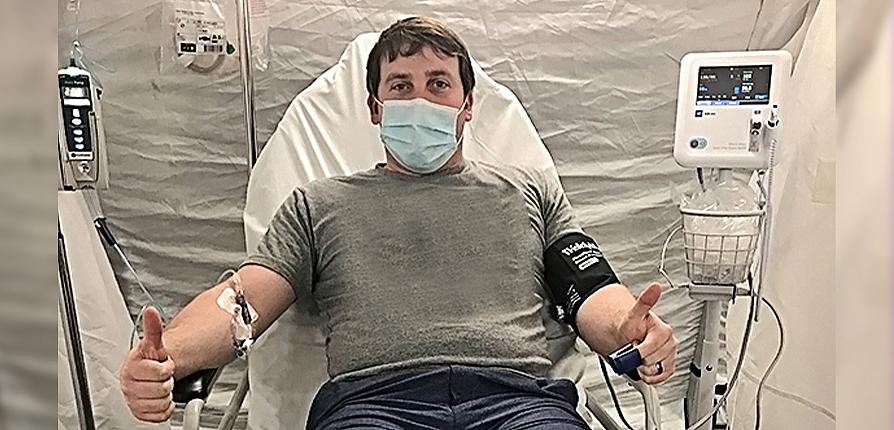
The Monday following Brandon's COVID-19 confirmation, I arrived at the hospital to be assessed for the REGN-COV2 trial. After some blood work and two more COVID-19 tests, I was randomized. By that evening, I was the first local patient to be enrolled in the study and receive four injections of either the trial agent, an antibody cocktail that targets two different sites of the spike protein found on the surface of the coronavirus SARS-CoV-2, or a placebo.
The next morning, Brandon received his infusion of either LY-CoV555, an investigational monoclonal antibody developed from the blood sample of a recovered COVID-19 patient, or a placebo.
Both trials were double-blinded, meaning neither the research team nor we knew if we received the trial agent or a placebo. However, we closely monitored our symptoms, and Brandon was required to keep a symptom log for the first month. He had to self-administer a COVID-19 test every evening, measure his temperature and log any symptoms he had or medications he took that day. He also had weekly blood draws during this time to monitor his body's response to the treatment agent.
For my trial, I attended weekly follow-up appointments for the first month to measure my vitals and conduct a COVID-19 test. Thankfully, I never tested positive for COVID-19. Brandon made a quick, full recovery, and his sense of smell and taste returned within a week.
While this experience was scary at first, ultimately, I would not trade it for anything. We were able to witness firsthand how incredibly passionate the physicians and researchers I am proud to call my colleagues are, not only for skillfully treating their patients but also for using their expertise to search for a therapy that will hopefully help so many affected by this illness.
During a time when all eyes are on research in the quest for a COVID-19 treatment or cure, I am grateful both my husband and I could participate in this global effort.
Top Image: Brandon Hawthorne's infusion of either Eli Lilly's investigational laboratory-made antibody drug or a placebo was delivered at the hospital and took nearly three hours.
 Axe ’Em, Jacks!
Axe ’Em, Jacks!